Transparent, enforceable regulations on human embryo models will benefit scientists and the public, argue A Foreman, S Franklin, E Jackson, C Rozeik, K K Niakan and LML Director Kathy Liddell.
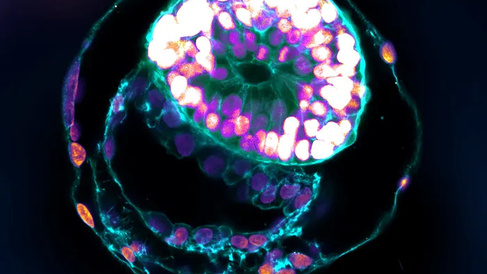
Integrated and non-integrated stem cell-based embryo models (‘SCB-EMs’) are becoming widely adopted tools in biomedical research with distinct advantages over animal models for studying human development. Although SCB-EMs have tremendous benefits for research, they raise a number of social, ethical and legal questions that affect future research and widespread adoption in industry and clinical settings.
Ethical Issues with Advanced SCB-EMs
The article discusses the advantages of using SCB-EMs over animal models for studying human development. The article highlights the ability of these models to recapitulate early human development, and to provide insights into the mechanisms of disease. The authors also discuss the different types of models, including integrated and non-integrated models, and their potential applications in research. They acknowledge, however, the challenges of using these models. If the similarities between the models and human embryos continue to develop, ethical issues will arise about oversight, accountability, and the duration and purpose of research studies. These issues will be particularly important if advanced human embryo models replicate primitive neural folds, early brain, blood islands, arm buds and early heart-like regions.
International Regulatory approaches to SCB-EMs
The 2021 International Society for Stem Cell Research (‘ISSCR’) Guidelines for Stem Cell Research and Clinical Translation provide helpful guidance on many of these issues, but do not have force in domestic law. Nevertheless, the article argues that by setting out a policy system, the guidelines are well-placed to catalyse national dialogues between scientists, regulators, experts and the public. A key issue is the application of the widely adopted 14-day rule for human embryo cultures to SCB-EMs.
The article highlights the importance of these guidelines in promoting responsible research practices through three avenues:
- Comparison with other national frameworks;
- adopting the policy content after extensive dialogue with the public and experts; and
- consensus-building within and beyond the scientific community.
Improving national governance for SCB-EMs: the UK and Australia as examples
The article also discusses the challenges and successes of the UK and Australian frameworks and highlights the importance of transparency, public engagement, and ethical considerations in their development. The authors argue that national frameworks are crucial to ensure the responsible use of these models in research and clinical settings and to promote public trust in science.
They emphasise the need to ensure the ethical and safe use of stem cell-based embryo models in biomedical research, stating that “careful appraisal and development of national legal and ethical frameworks is crucial. Paving the way to better regulation provides an ethical and social foundation to continue using human embryo models and to fully realise their potential benefits for reproductive medicine.”
This piece provides a comprehensive overview of the importance of national policy and governance review for stem cell-based embryo models in biomedical research.
The full article can be found here.
